The Innovative Medicines Initiative (IMI) is planning a substantial health blockchain project. The breadth of reach extends from the pharma supply chain and clinical trials through to health data. The Blockchain Enabled Healthcare program aims to partner nine big pharma companies with a consortium of stakeholders. In addition to the healthcare community and patients, the consortium will include SME blockchain companies and universities.
The IMI is a public-private partnership between the EU and the European pharmaceutical industry represented by EFPIA. For the Blockchain Enabled Healthcare program, the IMI is earmarking up to Euro 18m which is expected to last three years.
For any blockchain ecosystem, there’s a need to involve all the relevant stakeholders. Hence the plan to select a consortium to work with the pharma companies. The group is expected to include hospitals, clinics, health authorities, clinical labs, patient representatives as well as universities and SME blockchain companies.
These consortia will organize themselves into groups in order to apply to take part. The closing date for consortium applications is 24 October, and the IMI will announce the winning consortium in January 2019.
In the US there are multiple blockchain initiatives, including MediLedger and SAP that aim to comply with DSCSA legislation.
Aims
The IMI intends the blockchain program to have a broad impact. Patients should gain earlier access to the drugs they need, especially in developing countries. Plus they will be able to check the authenticity of their medication. In turn, this will clamp down on the counterfeit medication market which is estimated at Euro 160 billion a year.
Patients will also control their medical records but can grant access to their data for research purposes. That might include data from IoT devices and other information available through phone apps.
Automation will help healthcare providers including hospitals to operate more efficiently. Clinical trials will be easier to manage, and they’ll save money wasted on counterfeit or substandard drugs.
The pharmaceutical industry will gain from a reduced counterfeit market and greater supply chain efficiencies. By improving the clinical trials process and better access to data, there’s potential for increased innovation.
Finally, a decentralized network that shares data will alleviate some of the cyber threats that centralized storage systems suffer from.
Expected outcomes
The consortium will evaluate and design a series business use cases as well as assessing potential returns.
The program aims to build a foundation for ongoing work. So they’ll spend time and effort establishing healthcare blockchain standards. This alone is a substantial piece of work. Regarding clinical trials, this would encompass data quality and providing transparency to the patient. Also, it involves managing patient consent and recruiting people for drug trials.
Turning to the pharma supply chain, there will be standards for processes that enable product authentication, provenance, labeling, recalls and drug interaction.
Apart from standards, the consortium will formalize a governance model and identify all the compliance issues.
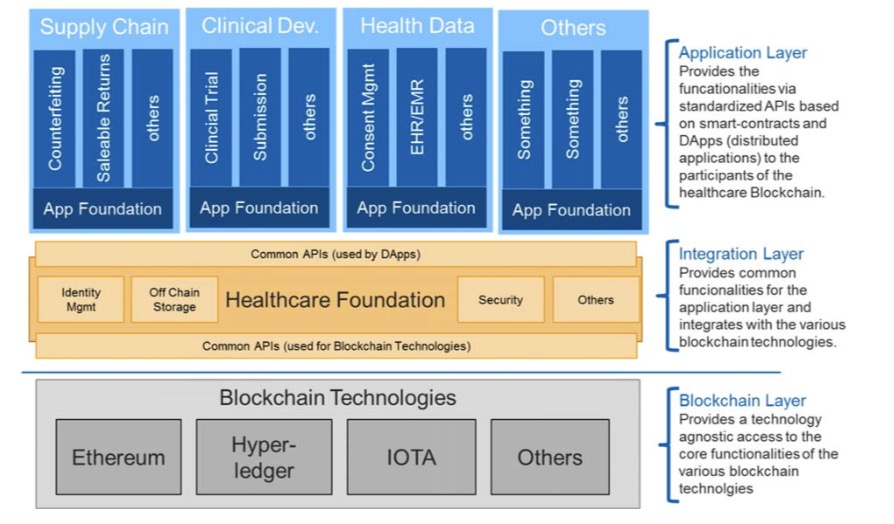
On the technology side, the consortium will produce an architectural framework that will encompass digital identity management, an efficient consensus mechanism, off-chain storage, global scalability, security, and high performance. Additionally, there will be a reference implementation that can assist in developing the selected use cases.
Finally, there’s the education and change management aspect to enable all stakeholders to go up the learning curve for both technical and organizational changes.
Full details for those planning a consortium are available from the IMI website.
If you’re interested in learning more about Blockchain and Pharma Supply Chain, Hanson Wade is running a conference in Boston in October. The link includes a 10% ticket discount. We’ve found Hanson Wade events have strong speakers, hardly any sponsor promotional content, and provide excellent networking opportunities.






